Empower Medical Device Growth
Denmark-based MedMarketExpand fuels growth for medical device companies in established and emerging markets. With over 20 years of expertise, a strong distributor network, and trusted partnerships with regulatory and clinical trial providers, we drive successful product launches, market expansion, and strategic sales across Europe and North America.
Our Services

New Markets
A solid business strategy does not always guarantee success. It's crucial to enhance your plans with a deep understanding of the local market—whether it's a country, region, or specific facility. Markets vary significantly in terms of access. Contact us to find out. That's how we can help.

Launch Strategy
With 20 years in the medical device industry, we’ve crafted international go-to-market strategies for both major companies and niche startups. While the path to success may twist and turn, the goal remains the same. Let us help you set a solid strategy and realistic goals.

Patient Engagement
Patient engagement in medical device development is the active involvement of patients in the design, testing, and feedback stages of creating a new device.
Involving patients throughout development not only ensures a more patient-centered product but also aids in regulatory approvals, as regulators increasingly prioritize evidence of patient engagement.

Regulatory Affairs & Documentation
Regulatory affairs in medical devices ensure products meet safety and compliance standards, navigating key frameworks like the FDA in the U.S. and MDR in Europe.
We support you in developing documentation and offer a streamlined quality management software solution that guides you efficiently throughout.

Clinical Trials
The importance of clinical evidence in demonstrating the safety and effectiveness of medical devices has never been greater. Allow us to guide you.
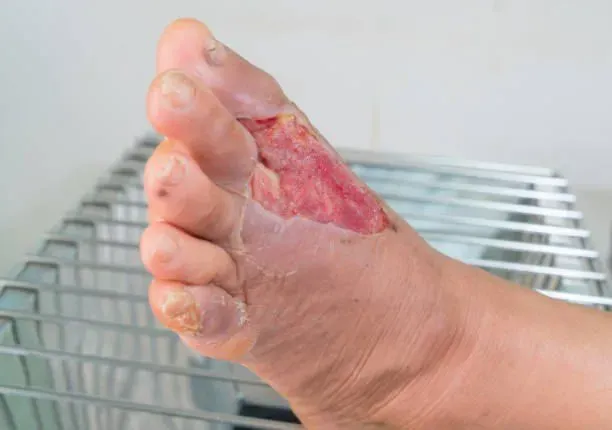
Wound Market Experts
Wound market experts offering services in clinical trials, market access, business development, and key opinion leader engagement.
We have a unique solution for ensuring your post market clinical data needs for wound treatment products - both acute and chronic.
We look forward to help!
Customers
Empowering innovation for a healthier tomorrow.
Join us in making a difference!
Contact us today

Ninette Neel Florboe Chislett, CEO and Founder
Ninette boasts over two decades of documented experience in the international life sciences sector, complemented by a decade in business leadership. Her expertise covers the entire product lifecycle, with roles in prominent firms including Mediq (MedTech Distribution Services), AXON Communications (Health and Clinical Trial Communications), Novo Nordisk (Pharmaceuticals), Coloplast (Medical Devices), and Cook Biotech (Biotechnology). Furthermore, Ninette has adeptly overseen operations in Canada, focusing on Medical Device Import and Distribution in the Long-Term/Elder Care market, and in Denmark, providing consultancy services to the Life Sciences Industry.




